
What is bi-metallic corrosion?
- Read time: 4 minutes
- Date: 21 Jun 2021
- Flat Roofing
- Living Roof
- Rainscreen & Façades
- Sheeting & Cladding
Bi-metallic corrosion occurs when two dissimilar metals come into contact with each other in the presence of an electrolyte, such as water. The interaction between the two metals, known as electrolysis, causes corrosion.
What is the science behind bi-metallic corrosion?
Bi-metallic corrosion, also known as galvanic corrosion, is where two different metals interact with each other causing corrosion. It is caused by an electrochemical occurrence that takes place when the two metals come into contact with each other in the presence of an electrolyte, such as rain, ground or seawater, causing an electrical reaction.
This electrical reaction causes one metal to become an anode (negatively charged) while the other, more noble (less corrosive) metal becomes the cathode (positively charged). An electrical current flows from the anode to the cathode, causing the anode to corrode at an accelerated rate.
What happens if bi-metallic corrosion occurs?
Corrosion weakens the chemical structure of the metal and can cause structural elements to fail in just a few years. This could have catastrophic results weakening the entire structure, putting the safety of the public at risk.

How can bi-metallic corrosion be prevented?
To minimise the risk of bi-metallic corrosion of fasteners, match the surface metal of the fastener with the metal it will be fixing. Where this is not possible it is best to match a large anode with a small cathode.
The smaller the surface area of the anode, relative to the surface area of the cathode, the more concentrated the flow of electrons at the anode and the faster the rate of corrosion. The larger the anode's surface area in relation to the cathode, the more spread out the flow of electrons will be and the slower the rate of the anode's corrosion.
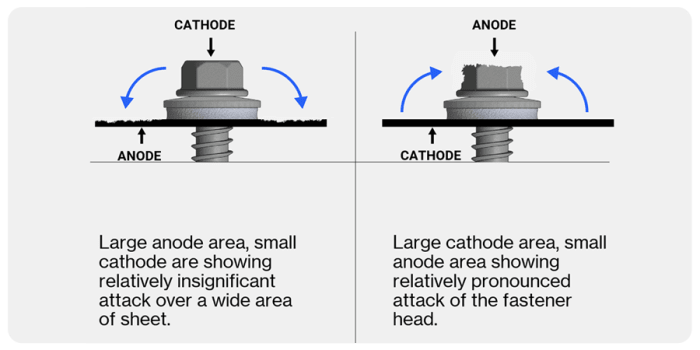
For example, aluminium is highly corrosive and will be an anode, whereas stainless steel is resistant to corrosion and is the cathode. Aluminium frames (large anode) should always be used with stainless steel fasteners (small cathode) to prevent bi-metallic corrosion.
Which metals are anodic and which are cathodic?
The Galvanic Series ranks metal according to their electrical potential in flowing seawater. The chart lists the most active metals (those that have the highest corrosion potential) at the top and the most noble (those that are passive and have the least corrosion potential) at the bottom. The order may change in different environments.
Anodic (Least Noble) + Active - High corrosion potential
Magnesium
Zinc
Aluminium
Carbon steel or cast iron
Copper alloys (brass, bronze)
Lead
STAINLESS STEEL
Nickel alloys
Titanium
Graphite
Cathodic (Most Noble) - Passive - Low corrosion potential





